
Organ-on-Chip Technology: Models, Market Advances, and Future Potential
Organ-on-chips (OoCs) have transformed the biomedical panorama. The numbers speak for themselves. In the last decade, 107 organs or substructures have been modeled using these devices, and OoCs publications have experienced 50% annual growth.
By simulating complex physiological environments, these systems seek to bridge the gap between conventional in vitro cell cultures and human clinical trials, offering a reliable and predictive alternative to animal testing for drug development and disease studies.
What Is Organ-on-Chip Technology and How Does It Work?
Organ-on-chips are engineered devices that use living cells in controlled environments to mimic human organ functions. These devices incorporate microfluidic channels lined with human cells that simulate blood flow, mechanical stimuli, and chemical gradients, enabling cells to behave as they would in vivo (Figure 1). The combination of cell diversity, extracellular matrix components, and dynamic fluid movement allows the mimicry of tissue-specific functions with high fidelity. Additionally, embedded biosensors provide real-time monitoring of pH, oxygen levels, and other parameters, facilitating detailed analysis of cellular responses.
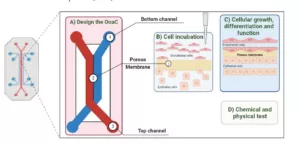
Figure 1. Key steps involved in the manufacturing process of an organ-on-chip. The method of producing different organ-on-chip is the same, considering its application. (A) The design of the platform must be in line with the properties that the system intends to address. (B) Different cells must be incubated in the device. (C) Cell growth, differentiation, and function are established so that the chip functions as an organ. (D) The data are obtained through tests that allow for changes to be detected in the system. Source: Morais AS, Mendes M, Cordeiro MA, Sousa JJ, Pais AC, Mihăilă SM, Vitorino C. Organ-on-a-Chip: Ubi sumus? Fundamentals and Design Aspects. Pharmaceutics. 2024 May 2;16(5):615.
This technology’s ability to reproduce organ physiology in a controlled, scalable format has transformed preclinical testing. Unlike static cell cultures, OoCs replicate critical tissue-tissue interfaces and biophysical forces such as shear stress, greatly enhancing biological relevance.
Organ-on-Chip Models and Their Biomedical Applications
Different OoC models have been designed to emulate various human organs, including the liver, lung, heart, gut, kidney, and brain. Gastrointestinal and neural organ-on-chip models dominate the field, evidenced by their leading publication volume and widespread use in research.
Each model serves a specific purpose, ranging from evaluating drug metabolism and toxicity to studying disease mechanisms. Their capacity to mimic organ-level physiology with high precision enables breakthroughs in several areas:
- Drug screening: OoCs support high-throughput testing of new molecules, helping identify promising drug candidates early in the development process. Platforms like liver-on-chip or multi-organ chips are particularly effective for evaluating pharmacokinetics and pharmacodynamics, accelerating decision-making in preclinical research.
- Drug response: These devices enable the observation of how human tissues respond to pharmaceutical compounds in real-time, capturing complex interactions such as drug absorption, metabolism, and toxicity. By simulating dynamic microenvironments, OoCs predict adverse effects and therapeutic outcomes more accurately than conventional models.

Figure 2. Schematic diagram depicting the multifaceted opportunities offered by OOC technology in the realms of drug response, personalized medicine, elucidation of disease mechanisms, and drug screening. Source: Srivastava SK, Foo GW, Aggarwal N, Chang MW. Organ-on-chip technology: Opportunities and challenges. Biotechnol Notes. 2024 Jan 5;5:8-12.
- Disease mechanism: By integrating patient-derived or genetically modified cells, OoCs can replicate disease-specific microenvironments, including cancer, neurodegenerative disorders, and respiratory infections. This enables the study of disease progression, mechanisms, and treatment strategies under conditions that closely resemble human pathophysiology.
- Personalized medicine: Using cells from individual patients, organ-on-chip technology can model personalized responses to drugs, offering a powerful tool for tailoring therapies. This approach holds particular promise in oncology and rare diseases, where treatment efficacy varies significantly between patients.
Organ-on-Chip vs. Animal Testing: Benefits and Limitations
OoCs offer significant advantages over animal models, including higher physiological relevance, reduced ethical concerns, and lower costs. Interspecies differences in disease pathways and gene expression profiles often lead to misleading results in drug screening. It is reflected in the high percentage, around 90%, of drugs that fail in clinical phases after showing promising results in the preclinical setting using animal models. OoCs can replicate human-specific responses that animals often fail to mimic, thereby improving drug safety predictions and reducing late-stage clinical failures.
However, challenges remain. The complexity of replicating inter-organ interactions, standardizing chip designs, and validating results across laboratories hinders widespread regulatory acceptance. There is a lack of universally accepted protocols and materials for OoCs design. There is also an urgent need to develop a blood mimetic, universal medium with nutrients and growth factors for the maintenance of different cell types. Addressing these organ-on-chip disadvantages is critical to ensuring reproducibility and scalability. Nevertheless, the field is progressing at an extraordinary pace, with a promising outlook for the continued development and impact of these devices.
Market Landscape: Leading Organ-on-Chip Companies and Innovations
The OoC market is rapidly expanding, driven by the need for alternatives to animal testing and the demand for more predictive preclinical models. Companies like Emulate, Mimetas, TissUse, and CN Bio Innovations have pioneered commercially available platforms for pharmaceutical and toxicological applications. These firms focus on scalability, integration with automated systems, and compatibility with high-throughput screening.
Regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are increasingly supporting the adoption of OoC systems, aligning with efforts to reduce animal testing. A key regulatory milestone accelerating adoption is the FDA Modernization Act 2.0, enacted in 2022. This legislation permits the use of specific alternatives, such as organ-on-chip, to animal testing to seek FDA exemptions for assessing drug safety and effectiveness during the preclinical phase. In April 2025, the FDA announced an action plan to phase out the animal testing requirement for monoclonal antibodies, with other drug types to follow.
This change not only validates organ-on-chip technology as a regulatory tool but also positions it at the forefront of efforts to modernize and streamline drug evaluation.
Future Directions: Integration with Organoids and Next-Gen Applications
The future of OoC technology lies in its convergence with organoid science. While organoids self-organize into 3D structures resembling native tissues, OoCs provide controlled microenvironments and real-time monitoring. Integrating these two technologies could yield even more physiologically accurate models, overcoming limitations such as necrosis in organoid cores or lack of vascularization.
Emerging trends include the development of body-on-a-chip systems that interconnect multiple organs to simulate whole-body physiology. These platforms hold promise for studying systemic diseases, personalized drug responses, and rare genetic disorders. Advances in biosensors, automation, and artificial intelligence (AI) will further enhance the analytical capabilities of OoCs, paving the way for their integration into clinical decision-making.
European efforts seek to shape the trajectory of organ-on‑chip technology. One prominent initiative is the UNLOOC (Unlocking data content of Organ‑on‑Chips) project. This three‑year €68 million programme brings together 51 partners across 10 countries, aims to develop scalable, AI‑powered multi‑sensor organ‑on‑chip platforms validated across five real‑world use cases, with applications spanning drug testing, disease modeling, cosmetics evaluation, and personalized therapies. BeCytes Biotechnologies is a proud partner. We isolate and provide lung and liver primary cells to the consortium for the development of these devices.
Organ-on-chip technology represents a transformative tool in the biomedical field, with the potential to revolutionize drug development, reduce reliance on animal models, and enable personalized medicine. As technical challenges are resolved and regulatory frameworks evolve, OOCs are poised to become indispensable in shaping the future of healthcare.
References
Mendes M, Morais AS, Carlos A, Sousa JJ, Pais AC, Mihăilă SM, Vitorino C. Organ-on-a-chip: Quo vademus? Applications and regulatory status. Colloids Surf B Biointerfaces. 2025 May;249:114507. doi: 10.1016/j.colsurfb.2025.114507.
Morais AS, Mendes M, Cordeiro MA, Sousa JJ, Pais AC, Mihăilă SM, Vitorino C. Organ-on-a-Chip: Ubi sumus? Fundamentals and Design Aspects. Pharmaceutics. 2024 May 2;16(5):615. doi: 10.3390/pharmaceutics16050615.
Shoji JY, Davis RP, Mummery CL, Krauss S. Global Literature Analysis of Organoid and Organ-on-Chip Research. Adv Healthc Mater. 2024 Aug;13(21):e2301067. doi: 10.1002/adhm.202301067.
Srivastava SK, Foo GW, Aggarwal N, Chang MW. Organ-on-chip technology: Opportunities and challenges. Biotechnol Notes. 2024 Jan 5;5:8-12. doi: 10.1016/j.biotno.2024.01.001.
Zushin PH, Mukherjee S, Wu JC. FDA Modernization Act 2.0: transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J Clin Invest. 2023 Nov 1;133(21):e175824. doi: 10.1172/JCI175824.






